
Growth and Division Control
How do cells grow and divide?Collective Cell Behaviour
How do cells collectively move within a dense tissue?Subcellular Mechanics
How do cells generate mechanical forces?Cellular Mechanosensing
How do cells sense their mechanical environment?
Overview | The goal of our research is to understand the mechanisms by which living cells and organisms autonomously generate forces, change shapes, grow, self-replicate and self-heal under applied stress. We integrate methods from statistical mechanics, soft matter physics and systems biology to develop quantitative models of biological function/behavior from molecular to organismic scales. Our approach is highly cross-disciplinary and is performed in close collaboration with experimental laboratories in phhysics and biology.





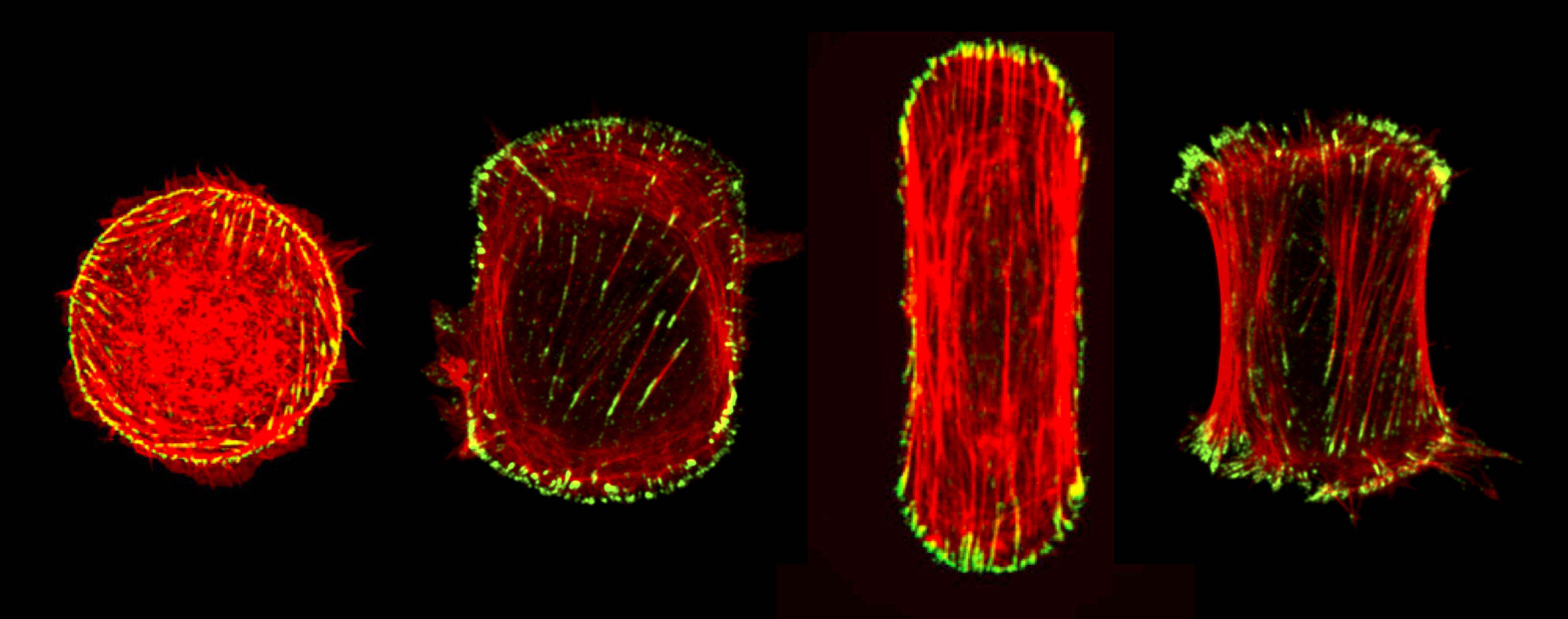
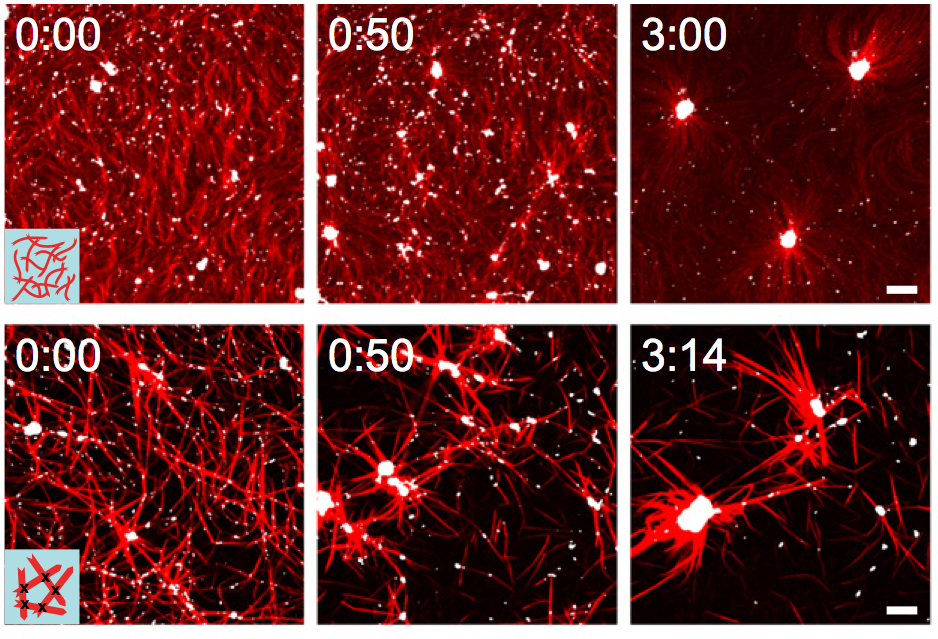
Design principles for subcellular organisation | Living cells are composed of a remarkable variety of subcellular structures with distinct architectures and mechanical properties tailored for specific biological functions. We seek to investigate how a cell constructs specific macromolecular structures with precisely organised components, from a finite pool of molecular building blocks. The actin cytoskeleton provides an ideal system for theoretical investigation of such a question, given the wealth of experimental information about its molecular structure, composition and dynamics. Despite this knowledge, it remains largely unknown how the component parts of the cytoskeleton work together to maintain robust micron-scale structures, while undergoing rapid turnover at the molecular scale.
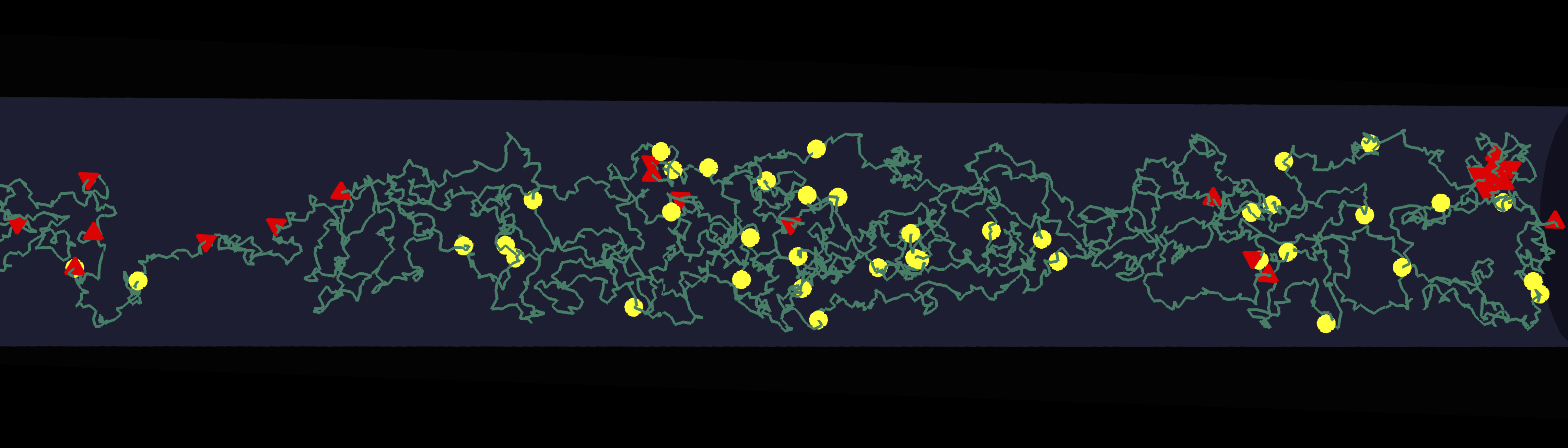
Mechanics and Statistics of cell growth, form and adaptation | Almost all living organisms maintain a narrow range of size distribution. This indicates that life has evolved to regulate cell division, so that cells can adapt to diverse growth conditions to maintain size homeostasis. Using bacteria as a model system, our research seeks to investigate the quantitative principles regulating cell size homeostasis, growth and division cycle. This has profound impacts for understanding the fitness of growing microbial communities in diverse environments, and in designing antibacterial strategies for fighting antibiotic-resistant infections.
Multicellular organisation and mechanics | While much is now known about the static and dynamic properties of individual cells and their cytoskeleton, far less is known about how single-cell level properties are integrated at multicellular scales for collective decision-making and tissue organisation. This is particularly important for tissue morphogenesis and repair, where cells collectively move, rearrange, proliferate or eliminate malignant neighbours for proper tissue formation. We seek to understand: (i) how cellular cytoskeletal machineries are integrated at the tissue level to pattern heterogeneous group of cells during organogenesis, (ii) how cells collectively respond to tissue loss after wounding through changes in mechanical properties, (iii) how interactions (mechanical and biochemical) at the single-cell level lead to shifts in the make-up of cell populations. To address these questions, we are developing cell-resolution models of multicellular assemblies that retain information on individual cell shapes, cell types and mechanical properties, while incorporating biochemical-mechanical feedback with cell polarity, actomyosin dynamics, and adhesions with the extracellular matrix.